TIGER advances cutting edge nanomedicine technologies for future off-the-shelf vaccines development for cancer.
eTheRNA’s drug technology platform consists of three main components
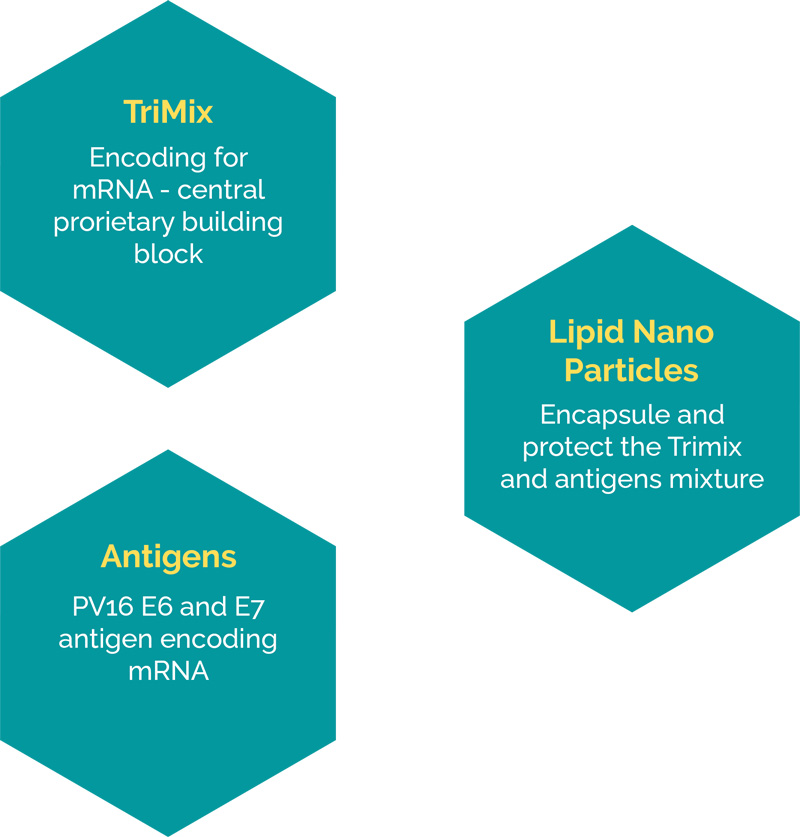
eTheRNA’s platform technology is applicable for many indications by simply exchanging the antigen-encoding payload for alternative TAA or TSAs.
